By Zachary Epperson, MLML Environmental Biotechnology Lab

Over the past few weeks, several marine mammals, particularly sea lions, have been exhibiting some haunting symptoms: writhing on the beach, bending back their necks, or lying suspiciously motionless. As the NOAA-NCCOS-funded, collaborative (MLML, UCSC, MBARI, USC, SCCWRP, UCLA, and IOOS) Ecology & Oceanography of Harmful Algal Blooms (ECOHAB) project gears up for its third week of sampling, data-armed scientists are ready with an explanation—toxic algae. Along with an armada of robotic labs and water quality surveillance vehicles roaming the bay, this field effort provides higher temporal and spatial resolution than our weekly shore based monitoring, which detected initiation of a mixed species Pseudo-nitzschia bloom in April (http://oceandatacenter.ucsc.edu/PhytoBlog/).
The diatom Pseudo-nitzschia spp. is known to produce the neurotoxin domoic acid (DA), responsible for cases of domoic acid poisoning (DAP, also known as amnesic shellfish poisoning), when contaminated tissue is consumed in high enough quantities. Symptoms of DAP may include vomiting, diarrhea, abdominal cramps, headache and dizziness; in severe cases the victim may experience difficulty breathing, confusion, disorientation, seizures, permanent loss of short‑term memory, coma and death. For this reason, recreational harvesting of shellfish is usually quarantined from about late April to Halloween.
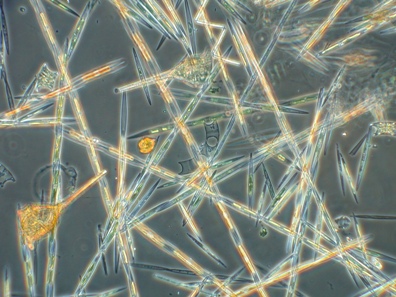
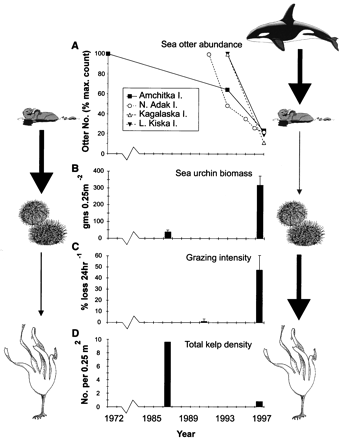
Though a spring bloom of Pseudo-nitzschia is typical, what’s surprising this year is the total DA load. According to Dr. Raphe Kudela (UCSC) levels this high haven’t been seen since the year 2000! And ECOHAB is out there to track it.
So what is MLML doing specifically, and what does this DA event mean for local marine life (and our clam chowder)? Over the past two weeks, MLML’s Environmental Biotechnology Laboratory (EBL), in collaboration with UCSC and USC research teams, has been sampling twice a week in Monterey Bay from the R/V John H. Martin (MLML). Each day, water is sampled at multiple stations using MLML’s new conductivity, temperature, and depth (CTD) rosette profiler. Water samples are taken from depths of interest, which is then processed and analyzed on board and back at the lab. By making these measurements and data sets, researchers hope to elucidate bloom and toxin dynamics and processes. One recently constructed product of this research, made by the Central and Northern California Ocean Observing System (CENCOOS), is an interactive and predictive model for Pseudo-nitzschia blooms and DA production, which can be accessed online (http://www.cencoos.org/data/models/habs). As a MLML student, it’s been fascinating to see the practicality of our coursework in this research. Classes like Biological and Chemical Oceanography draw immediate connections to the concepts and sampling methods used in this study. Due to the irregularity of a bloom this toxic, it’s also been interesting to see how multiple research teams snap into action, processing samples all week and weekend, and collaboratively discussing and tracking the bloom’s behavior and dynamics. What’s more, the timing of our ECOHAB cruise this spring is nothing short of impeccable; we’ve been able to track this bloom virtually from it’s beginning with incredible resolution!

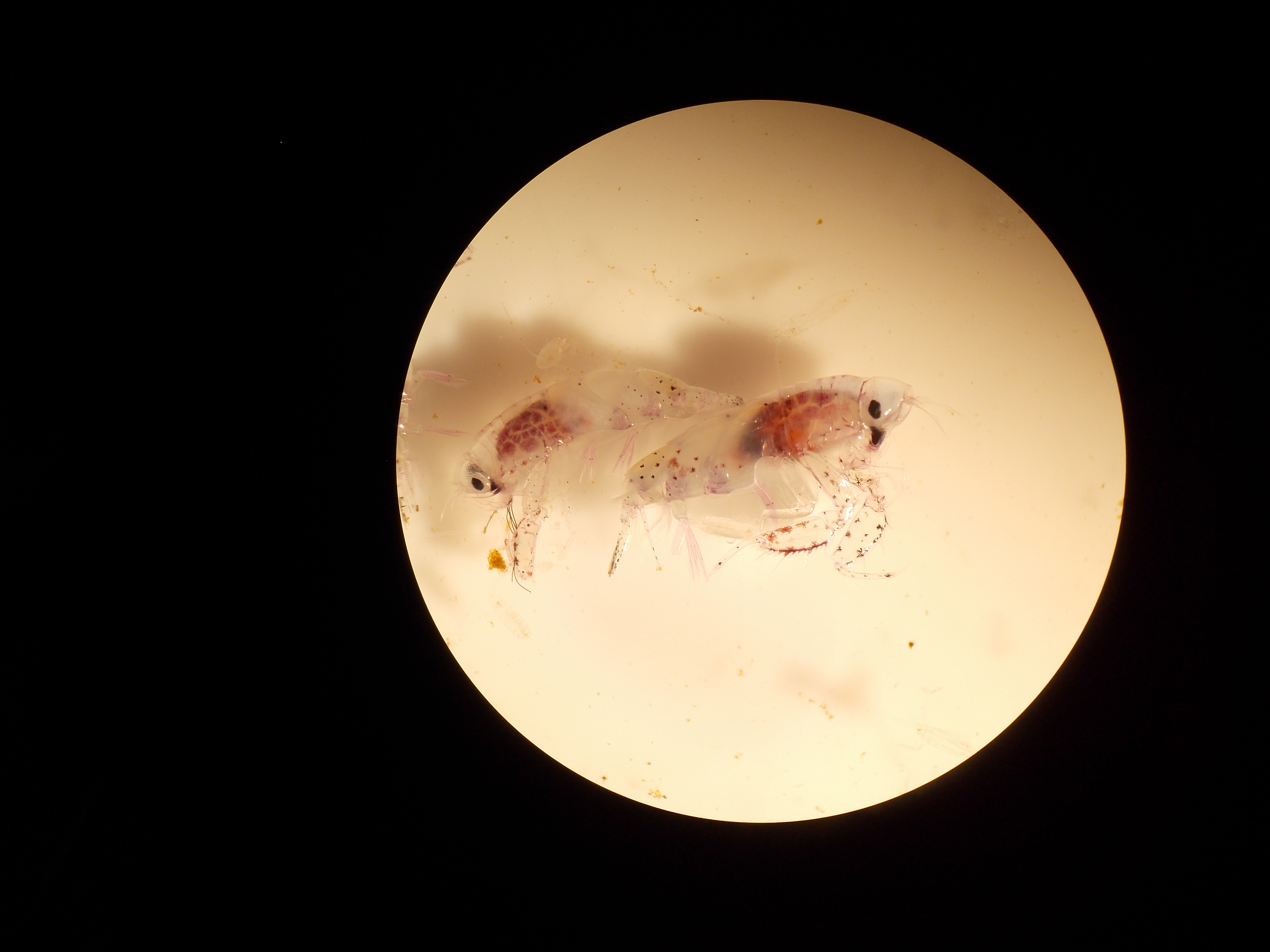
As regards our seafood diet, DA tissue levels in shellfish, particularly mussels, are unsafe for recreational harvesting and consumption (note that commercially harvested shellfish are only sold after passing food safety standards, and can be trusted.) A rockfish tissue sample collected from Stillwater Cove showed detectable levels of DA, as have Monterey Bay squid, but these do not currently pose a human health threat being below the regulatory limit of 20 ppm (μg DA/g tissue). In terms of the ecosystem, DA has been detected throughout the food web and will likely have biomagnifying effects, especially if the bloom continues and DA levels remain high.
In the end, more samples need to be taken to better capture the effect of this massive event. EBL will be conducting zooplankton tows during our final cruises to assess DA levels in primary consumers and hopes to collect sand crab samples from Monterey beaches (san crabs have been collected from the MBARI-MLML Norte beach with toxic DA tissue loads.) Of course, a key interest is the piscine and piscivorous food webs. Benthic fishes, especially flatfish are needed to assess the ecosystem. If anyone is interested, EBL will gladly accept any specimens from local divers or fisherman. Whole body is best, but not required. A sample’s date caught and location would also be appreciated. We are located across from small boats in the Norte facility (7544 Sandholdt Rd, Rm 36). We’ll be accepting specimens over the next few weeks.













 The Marine Operations Building (aka the Firehouse) has been a busy place this morning. The Marine Environmental Studies of the Gulf of California class is staring their journey toward La Paz, Mexico today, eventually landing on a small island called Isla Partida just north of La Paz. Here they will conduct a variety of field research projects including sea floor mapping, fish grazing and artisanal fishing studies as well as fish, seaweed and invertebrate surveys. Check back in a few weeks for a more detailed account of their adventures!
The Marine Operations Building (aka the Firehouse) has been a busy place this morning. The Marine Environmental Studies of the Gulf of California class is staring their journey toward La Paz, Mexico today, eventually landing on a small island called Isla Partida just north of La Paz. Here they will conduct a variety of field research projects including sea floor mapping, fish grazing and artisanal fishing studies as well as fish, seaweed and invertebrate surveys. Check back in a few weeks for a more detailed account of their adventures!