By Alex Olson & Holly Chiswell, Chemical Oceanography

On June 5th, members of the Marine Pollutions Studies and Chemical Oceanography Labs under the direction of Dr. Kenneth Coale, began a week-long journey on the R/V Point Sur to investigate the recent findings of mercury in coastal marine fog. Dubbed “The Fog Cruise”, the crew and science party aboard sampled near and offshore waters using oceanographic tools for signs of methylmercury (MeHg), from deep sea sediments to fog above the sea surface.
Mercury (Hg) is a naturally occurring element and has various chemical species, with monomethylmercury (MMHg) and dimethylmercury (DMHg) being forms of the neurotoxic MeHg species. Despite femtomolar (parts per trillion) levels in the ocean, MeHg is bioavailable and bioaccumulates up the food chain where humans are readily exposed to dangerous levels. Solving where and how MeHg is created (where additions of methyl, CH3, take place) in the open ocean and transferred into coastal fog would be filling a critical gap in our knowledge of the mercury cycle (below).
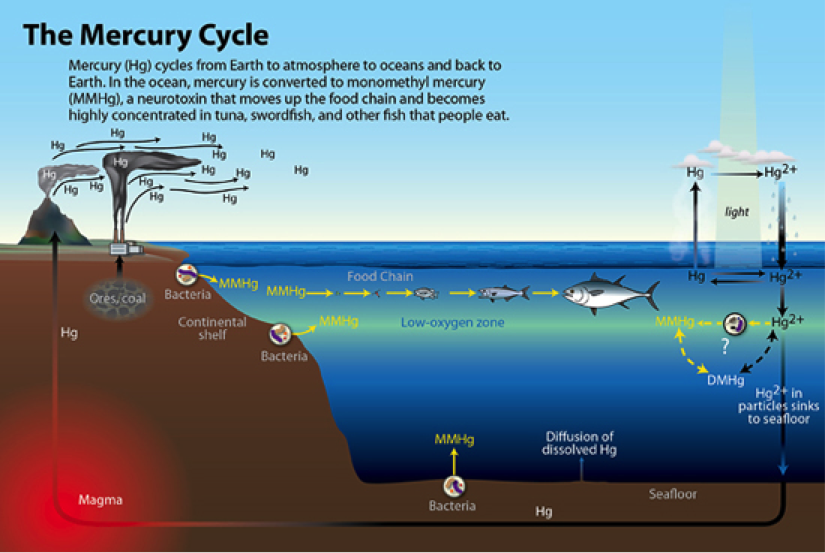
Hg is a widespread and common element on Earth, volcanism being a large natural source. As the cycle above indicates, Hg is versatile. In mineral phase it is found as a gas, liquid and solid. What makes this abundant element difficult to track is it’s ability to transform into species with different properties. Through just a few reactions, it can move from a water soluble species to a hydrophilic fat soluble species, and then with the right amount of sunlight, wisps in the atmosphere to await it’s next evolution. Sampling Hg necessarily requires a variety of collection and analytical methods in order to comprehensively understand its movement through environments.

Many have traveled on the R/V Point Sur, but no one has traveled on the R/V Point Sur with a giant fog tower attached to the forward deck….until now. Fog towers are maintained on land and have reported collecting substantial amounts of sample, so the idea was to create one for the marine side. With a little help from the Santa Cruz County Amateur Radio Club and their donation of the tower segments, the team erected this tower to collect samples of marine fog for traces of mercury. We deployed the tower at night and took it down in the morning to anazlyze any fog it collected. Unfortunately, the tower did not collect as much volume as we anticipated, but what we got was enough for a small mercury signal.
On our CTD rosette (conductivity, temperature, depth), mounted sensors provide a real time profile of oxygen, temperature, salinity and other parameters to visualize the water column, and where to collect water. The large bottles that make the CTD rosette (niskin bottles) are then tripped to close at the desired depths by the R/V Point Sur’s marine technician, Stian Alesandrini, a 12 year veteran of Antarctic research. In his office (also called the E-lab) there is a “meeting of the minds” between Stian and Kenneth to discuss what depths samples would be needed based on the CTD sensors data. The term “Etch-a-Sketch Oceanography”(TM) is thrown around during the cruise after a few interesting profiles. The analysis of this water yields a chart showing DMHg and total Hg concentrations with depth.

We also took a biological approach to finding sources and sinks of the different forms of mercury. We used two different phytoplankton nets, one that sank and gave us the phytoplankton at depth, and a neuston net, engineered with a pool noodle in order for the net to sample the neuston, which is the surface of the ocean. The neuston samples were especially important to conduct as they can indicate fluxes of mercury across the air-sea interface. As we were conducting the tows and bringing the nets back on the stern, we could immediately tell if the phytoplankton and neuston samples were going to be healthy. The general trend was that the nets from the stations closer to shore came up with samples that were “chocolate” in color, thick and smelling like productivity. We found that the offshore site samples were full of healthy diatoms, unbroken chains of cells as and not many broken cells, but the mass of diatoms in total was not as much. Analysis of these critters included chlorophyll and identification shipboard. The samples from the stations were filtered and will be tested for amounts of mercury in the lab. The sites also took zooplankton tows usually at 20 meters for 5 minutes. This place in the water column was determined by the fluorescence reading from the CTD cast that would happen prior to this tow at the station. Generally the zooplankton tows would give us a lot of jellies, ctenophores, and dolliolids.

A multi-core (seen below) was dropped onto soft bottom seafloor sites to capture potential fluxes of MeHg into the overlying bottom water. Select cores were incubated for 24hrs and analyzed for changes in MeHg content to investigate potential flux from sediments into the overlying bottom water.


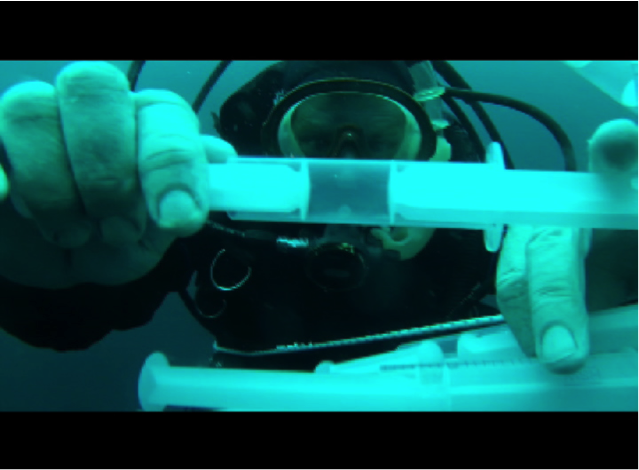
A former professor likened the mass collection from trawler surveys and plankton tows to dragging a half mile wide net through San Francisco from an airplane. While you might get a goodie bag of city denizens (cars, garbage cans, people, lamp posts, trees, squirrels, feral cats, etc.) you wouldn’t know how they all interact. You’d end up with questions like “Do people live in the garbage cans?” Blue water diving provides another window into open ocean dynamics. It allows us to see what CTD rosette, plankton tows (large bag filter), and video sampling methods cannot, an unsquished and complete in situ perspective. CTDs and nets can damage small organisms; while video and mechanical collectors can be clumsy. The intuition and dexterity of a human diver at depth can be far more instrumental in collecting fragile small-bodied plankton than any other method. Using modified plastic syringes, divers can suck in likely planktonic suspects for MeHg analysis.

MPSL analysts Amy Byington and Autumn Bonnema ran multitudes of water samples, literally day and night during the cruise, for DMHg and elemental Hg, both of which are typically in gas form. Their gas state gives them a short 3-4 hour sample viability, necessitating their on-board analysis. For MMHg and total Hg analyses, sediments, plankton, and water samples require further preparation and processing back at MPSL. It’s also a lot easier to analyze without large swells, 50-knot winds and notebooks that stay on the counter.

Our initial shipboard DMHg and elemental mercury results demonstrated profiles similar to other nutrients in the ocean. This means that the concentrations increased with depth, a typical indication of uptake by phyto- and zooplankton. It is possible that since DMHg and elemental Hg are dissolved gases in seawater, they are presumably escaping into the atmosphere near the surface, which could explain their nutrient-like profiles. If the surface waters show comparable concentrations of MMHg to the dissipating DMHg, we can assume that the DMHg has not completely offgassed but instead changed forms to MMHg. If we find an insignificant MMHg signal in the area of DMHg depletion, then atmospheric offgassing is the likely sink of DMHg in surface water. The work ahead will be dominated by these MMHg analyses, with the results informing us of general trends. In theory, these trends will help narrow our search and sampling on the next “Fog Cruise” in August. The challenge now is to finish analyzing before the follow-up cruise in August! Tune in for our next update!


