by Liz Lam, Biological Oceanography Lab

Ballast water treatment and testing is a big focus here in the Biological Oceanography lab, and this is no exception even when it comes to class projects. Last semester, I started a project aiming to improve one of our counting techniques. I’d previously written about IMO’s restriction to 10 organisms per 1,000 liters of discharged ballast water and counting zooplankton under a microscope in order to check for these results. But when it comes to even smaller organisms, such as algae and other even tinier phytoplankton, different methods are called for.
We already have a pretty clever way of quantifying such microscopic organisms by using a few chemical and optical tricks. The first key ingredient is fluorescein diacetate, or FDA. One of the special features of this molecule is that it can only be cleaved by certain proteins in live cells. Once FDA is split, what remains is fluorescein, a compound that glows bright green when excited under blue light. We can then use an epifluorescence microscope to both shine the right wavelength of light and magnify a sample in order to count any green organisms. If it glows green, then it means it’s alive! This allows us to quantify the number of live organisms that are extremely small and difficult to see.

Unfortunately, even such a clever method has a few key disadvantages. First of all, these water samples must be counted in a 3D well-plate, making it very difficult to find organisms at different depths. This is like trying to count chickpeas in an Olympic sized swimming pool! Secondly, fluorescein eventually leaks out of cells, so these samples have to be counted immediately after they’ve been treated and they can’t be preserved over time. That’s a bit too time-consuming and inconvenient for ballast treatment testers.
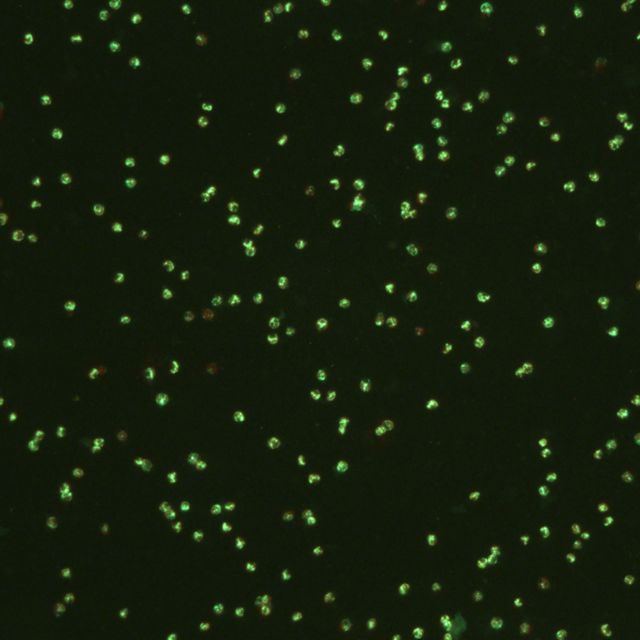
What I investigated at the end of last semester is the possibility of preparing samples on flat slides. This would eliminate the depth-of-focus issue and as a bonus, allow us to take photographs of known volumes of samples. I also experimented with a variety of fixation methods, or ways of preserving the fluorescein inside cells so that it would stay there for an extended period of time. Surprisingly, microwaving the slides seemed to do a fairly good job of keeping the fluorescence within the cells. These findings have given me an exciting jumping-off point for this semester!

