Why Study Trace Metal and Nutrient Cycling in the Ocean?
Phytoplankton, the microscopic plant-like organisms that sit at the base of the marine food web, account for about half of the primary production on Earth and contribute to the removal of carbon dioxide from the atmosphere. In order to grow, phytoplankton need to acquire major nutrients (C, N, P and Si) as well as a suite of trace metals (e.g., Fe, Zn, Co, Cu, Mn) that are generally present at vanishingly low concentrations in surface seawater but are essential to phytoplankton metabolism. Iron, for example, makes up only a billionth of the dissolved mass of seawater but limits the growth of phytoplankton in about a third of the oceans because its concentration is unable to meet the needs of marine phytoplankton. On the other hand, some metals (e.g., Zn, Cu, Cd), while essential at low concentrations, can become toxic to marine life at elevated concentrations, particularly in coastal waters influenced by human activities.
Studying the distribution and cycling of trace metals and nutrients in the marine environment is thus of fundamental importance, because they influence marine productivity, the species composition of phytoplankton communities and, ultimately, global carbon cycling.
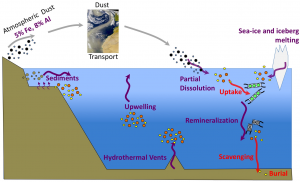
Trace metals are challenging to study because of their complex chemistries and extremely low levels in the marine environment. While great progress has been made in the understanding of metal distributions in coastal and open-ocean systems, their sources, sinks and internal cycling are still weakly constrained and significant analytical challenges remain to enable long-term in situ measurements.



