Our study of phytoplankton associated with sinking particles was recently published in the journal Limnology and Oceanography. (link to open access publication)
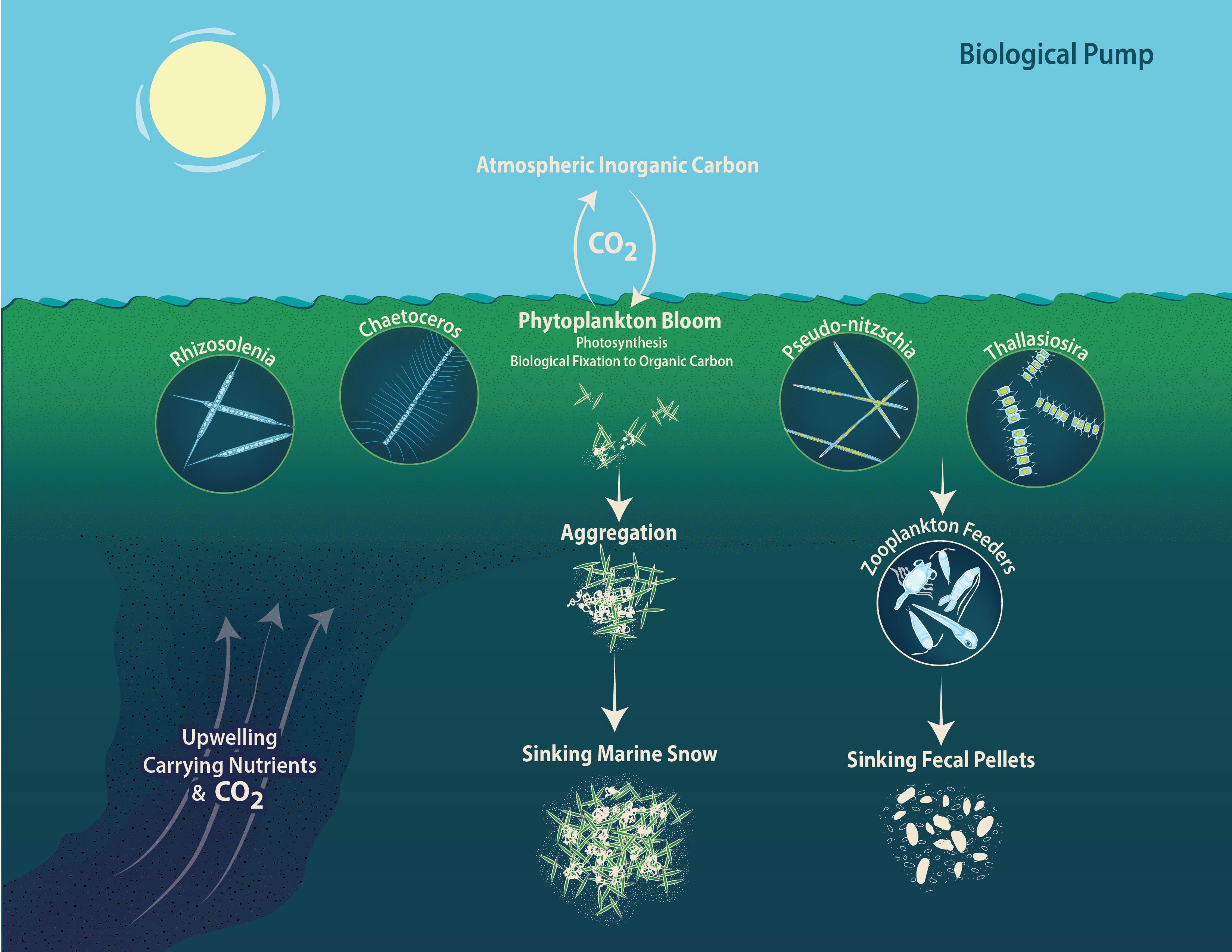
The amount of carbon that sinks out of the surface ocean in the form of organic particles is highly variable and difficult to predict, in part because the ecological processes that lead to the export of these particles are complicated. In this study, we attempted to resolve the mechanism of particle export across a large ocean basin, with a special focus on phytoplankton cells.
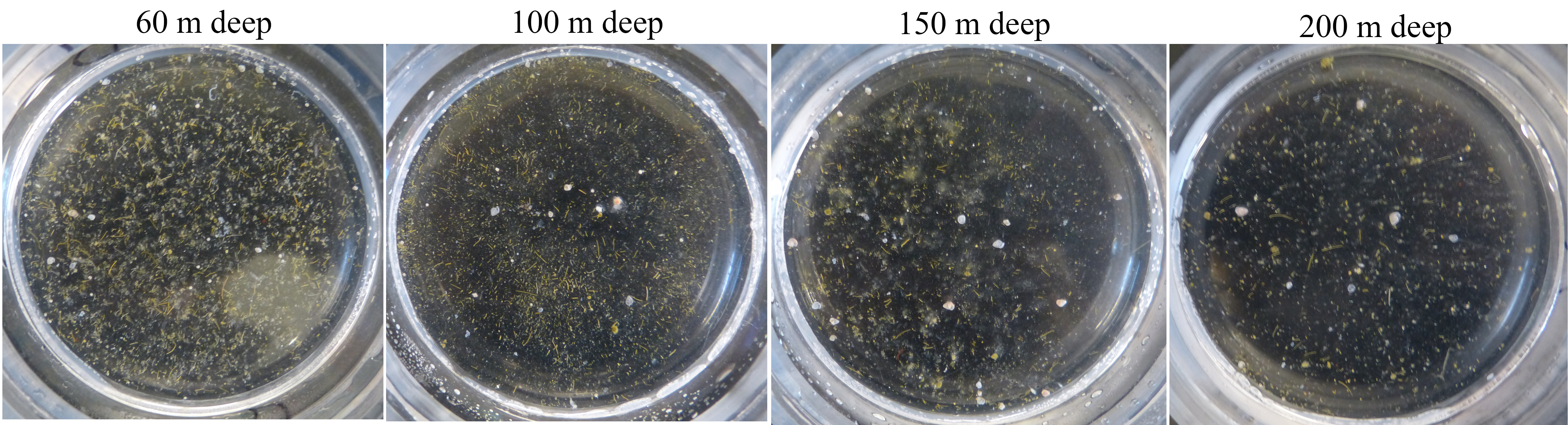
T his data was collected in 2013 during a 45 day research cruise on the R/V Knorr (aka: the DeepDOM cruise). We sailed from Uruguay to Barbados and deployed sediment traps all along the way (see locations on the map). You can see a video about this project here, and on the “Science Videos” page.
his data was collected in 2013 during a 45 day research cruise on the R/V Knorr (aka: the DeepDOM cruise). We sailed from Uruguay to Barbados and deployed sediment traps all along the way (see locations on the map). You can see a video about this project here, and on the “Science Videos” page.
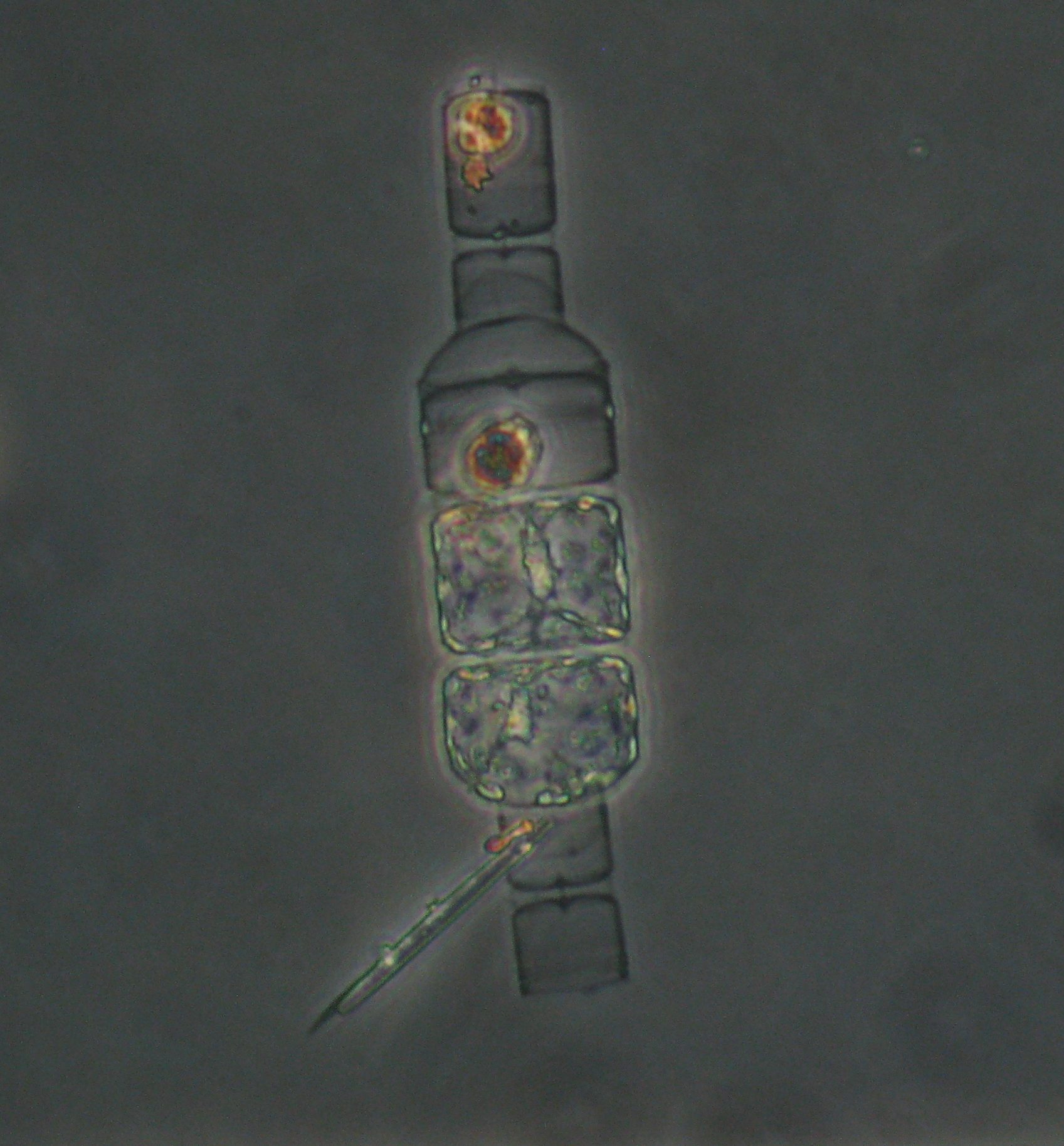
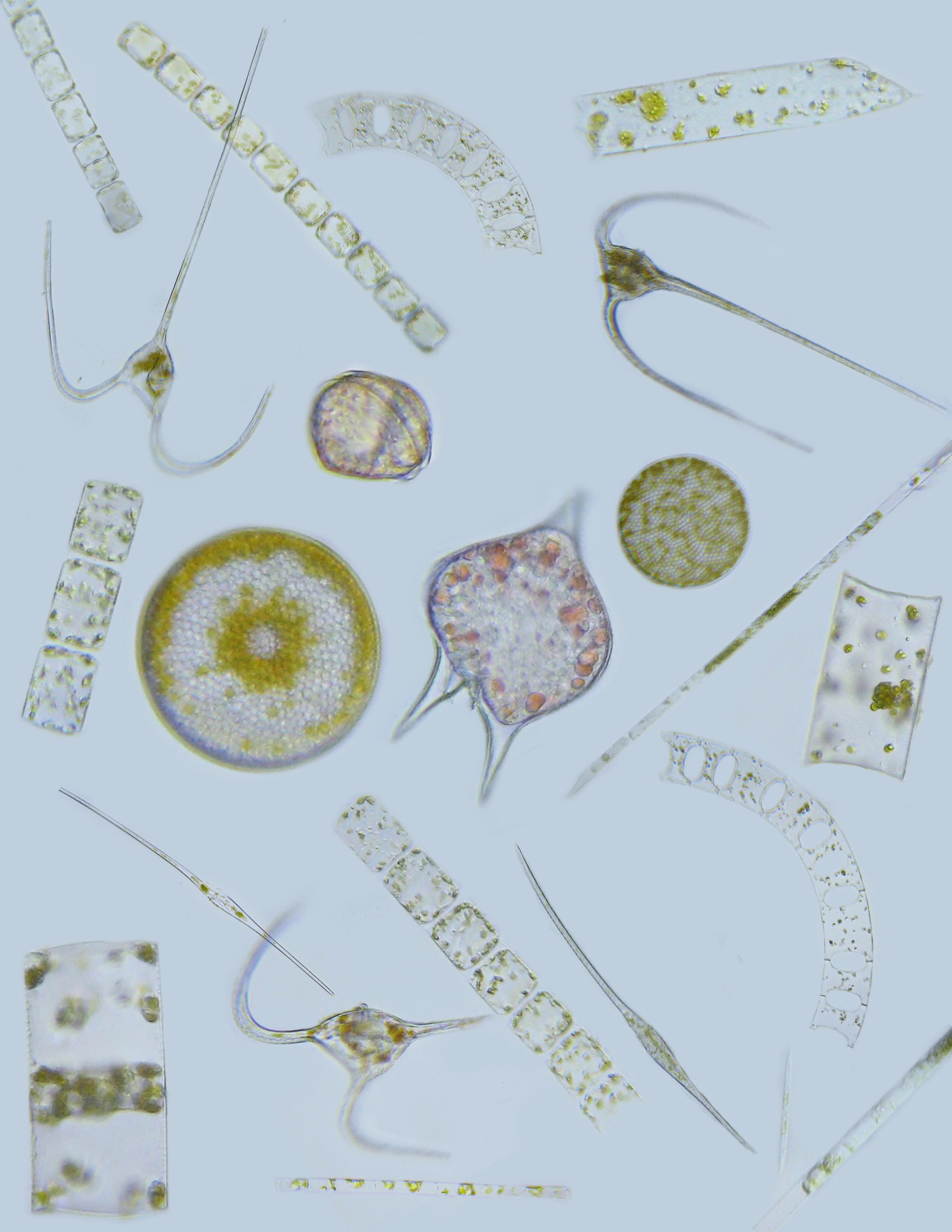
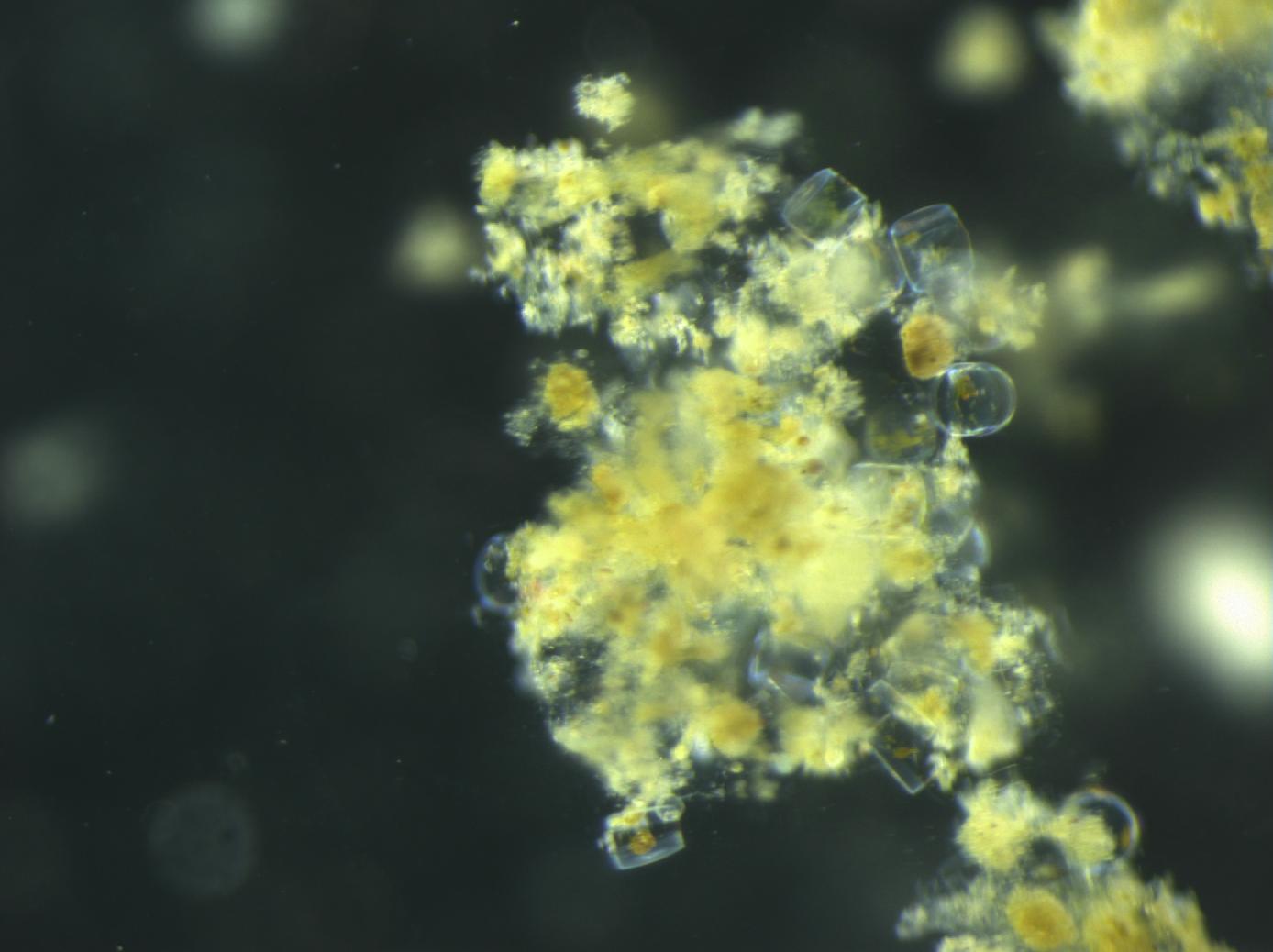
I used sediment traps containing a gel layer at the bottom to resolve the identity of individual particles and cells sinking out of the surface ocean (see micrographs in the map figure). The observation that excited me the most was the presence of individual phytoplankton cells in the gel layer. How could such tiny “particles” sink out of the surface ocean? We used a statistical approach to hypothesize how these tiny, slow-sinking cells were transported out of the sunlit surface ocean where they grow.
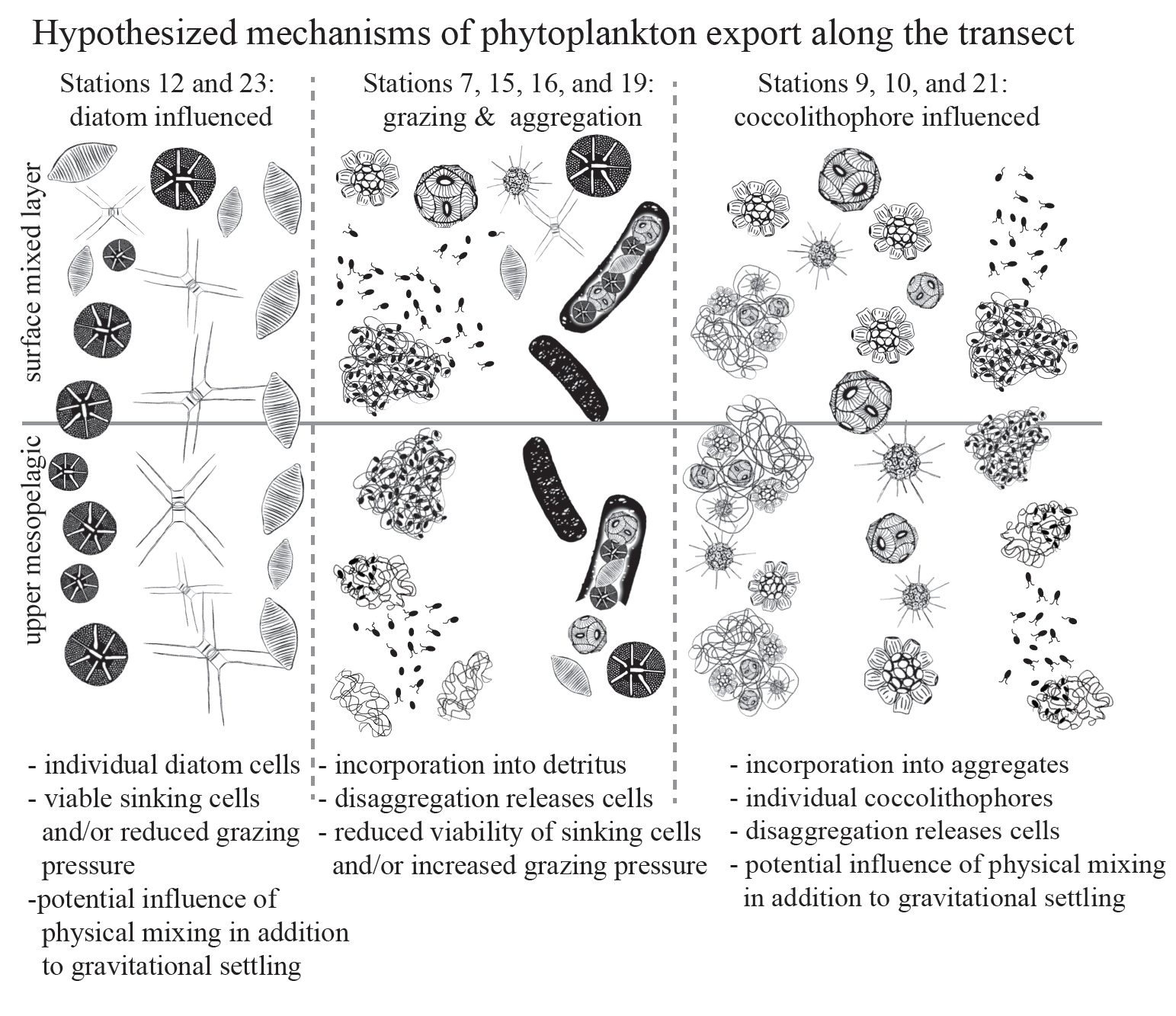
At most locations, phytoplankton appeared to be associated with sinking fecal pellets and aggregates; they were most likely carried along with these fast sinking detrital particles. However, at several locations sinking phytoplankton cells did not seem to be associated with detrital particles. At two locations, intact and living diatoms appeared to be sinking by themselves, suggesting that they could sink fast enough to be transported out of the surface ocean. Alternatively, they may have been transported by physical mixing. Coccolithophores were particularly dominant at 3 of the observed locations, and we hypothesize that they were either transported by sinking, physical mixing, or in association with detrital aggregates.
We hope that these methods will continue to resolve the mechanisms of “the biological pump” in future studies. With a better understanding of the types of particles responsible for carbon uptake by the ocean, and mechanisms responsible for their transport to the deep sea, the global carbon cycle can be better quantified.
This project was a collaboration with Ben Van Mooy (WHOI), Sonya Dyhrman (Columbia/LDEO), and Ken Buesseler (WHOI), who are also coauthors.